Are you infectious if you have a positive PCR test result for COVID-19
Source : CEBM – The Centre for Evidence-Based Medicine develops, promotes and disseminates better evidence for healthcare.
August 5, 2020
Tom Jefferson, Carl Heneghan, Elizabeth Spencer, Jon Brassey
PCR detection of viruses is helpful so long as its accuracy can be understood: it offers the capacity to detect RNA in minute quantities, but whether that RNA represents infectious virus may not be clear.
During our Open Evidence Review of oral-fecal transmission of Covid-19, we noticed how few studies had attempted or reported culturing live SARS-CoV-2 virus from human samples.
This surprised us, as viral culture is regarded as a gold standard or reference test against which any diagnostic index test for viruses must be measured and calibrated, to understand the predictive properties of that test. In viral culture, viruses are injected in the laboratory cell lines to see if they cause cell damage and death, thus releasing a whole set of new viruses that can go on to infect other cells.
We, therefore, reviewed the evidence from studies reporting data on viral culture or isolation as well as reverse transcriptase-polymerase chain reaction (RT-PCR), to understand more about how the PCR results reflect infectivity.
Viral cultures for COVID-19 infectivity assessment. Systematic review. Tom Jefferson, Elizabeth Spencer, Jon Brassey, Carl Heneghan medRxiv 2020.08.04.20167932; doi: https://doi.org/10.1101/2020.08.04.20167932
What did we find?
We searched for studies that reported culture or isolation of SARS-CoV-2 using samples from Covid-19 patients.
We identified fourteen studies that succeeded in culturing or observing tissue invasion by SARS-CoV from various samples from patients diagnosed with Covid-19. The quality of these studies was moderate with a lack of protocols, standardised methods and reporting.
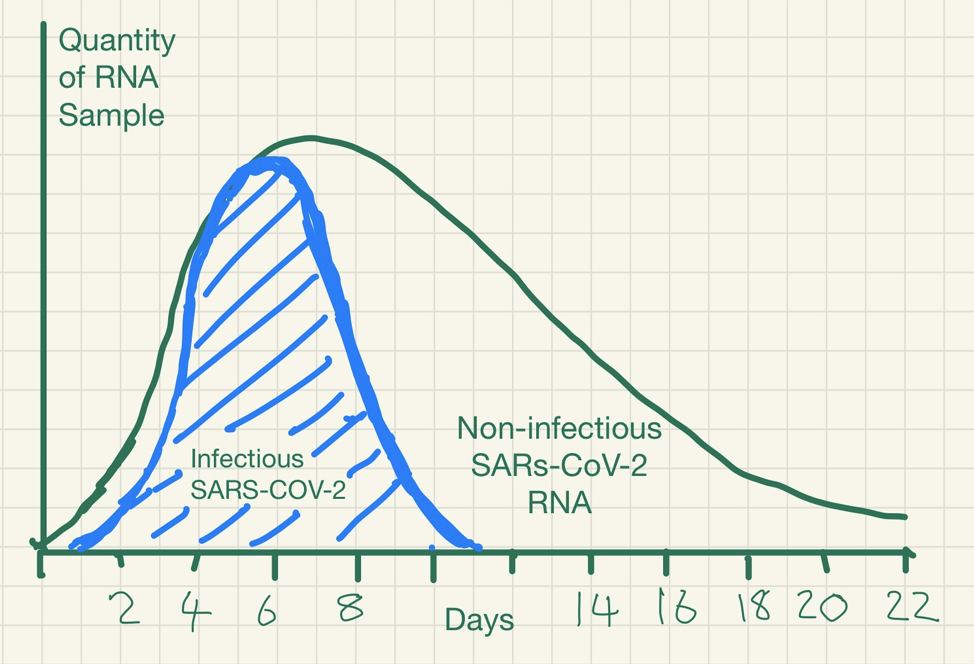
Data are sparse on how the PCR results relate to viral culture results. There is some evidence of a relationship between the time from collection of a specimen to test, symptom severity and the chances that someone is infectious.
One of the studies we found (Bullard et al) investigated viral culture in samples from a group of patients and compared the results with PCR testing data and time of their symptom onset.
The figure below reported in Bullard shows how the probability of SARS-CoV-2 infectious virus is greater (the red bars) when the cycle threshold is lower (the blue line) and when symptoms to test time is shorter – beyond 8 days, no live virus was detected.

Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants medRxiv 2020.06.08.20125310.
Kampen and colleagues studied the shedding of infectious virus in 129 hospitalized patients with COVID-19. The duration of infectious virus shedding ranged from 0 to 20 days post-onset of symptoms, and the probability of detecting infectious virus dropped below 5% after 15 days post-onset of symptoms. They also report that the amount of virus is associated with the detection of infectious SARS-CoV-2, and once neutralizing antibodies are detected in the serum the virus becomes non-infectious.
When the samples were taken seemed important for viral culture. In a case report, SARS-CoV-2 RT-PCR continued to detect the virus until the 63rd day after symptom onset whereas the virus could only be isolated from respiratory specimens collected within the first 18 days. In a cohort of 59 patients, fecal discharge was longer after respiratory shedding stopped. Gupta et al. w15 reported the duration for fecal shedding of viral RNA after clearance of respiratory samples ranged from 1 to 33 days and in one patient was up to 47 days from symptom onset.
It was not possible to make a precise quantitative assessment of the association between RT-PCR results and the success rate of viral culture within these studies. These studies were not adequately sized nor performed in a sufficiently standardised manner and may be subject to reporting bias.
Furthermore, context matters. The cycle threshold level for detecting live virus will vary by setting (hospital vs. community); depending on the symptom severity and the duration of symptoms, as well as the quality of the testing. Cycle thresholds are the times that the amplifying test has to be repeated to get a positive result. The higher the viral concentration the lower amplification cycles are necessary.
Why does the cycle threshold cut-off matter?
RT-PCR uses an enzyme called reverse transcriptase to change a specific piece of RNA into a matching piece of DNA. The PCR then amplifies the DNA exponentially, by doubling the number of molecules time and again. A fluorescent signal can be attached to the copies of the DNA, and a test is considered positive when the fluorescent signal is amplified sufficiently to be detectable.
The cycle threshold (referred to as the Ct value) is the number of amplification cycles required for the fluorescent signal to cross a certain threshold. This allows very small samples of RNA to be amplified and detected.

The lower the cycle threshold level the greater the amount of RNA (genetic material) there is in the sample. The higher the cycle number, the less RNA there is in the sample.
What does this mean?
This detection problem is ubiquitous for RNA viruses detection. SARS-CoV, MERS, Influenza Ebola and Zika viral RNA can be detected long after the disappearance of the infectious virus.
The immune system works to neutralise the virus and prevent further infection. Whilst an infectious stage may last a week or so, because inactivated RNA degrades slowly over time it may still be detected many weeks after infectiousness has dissipated.

PCR detection of viruses is helpful so long as its limitations are understood; while it detects RNA in minute quantities, caution needs to be applied to the results as it often does not detect infectious virus.
What can we conclude?
These studies provided limited data of variable quality that PCR results per se are unlikely to predict viral culture from human samples. Insufficient attention may have been paid how PCR results relate to disease. The relation with infectiousness is unclear and more data are needed on this.
If this is not understood, PCR results may lead to restrictions for large groups of people who do not present an infection risk.
The results indicate that viral RNA load cut-offs should be used: to understand who is infectious, the extent of any outbreak and for controlling transmission.
What next?
Our review is an Open Evidence Review. We will update the findings as additional evidence becomes available. We submitted the manuscript to the preprint server MedRxiv. (see here) We will continue to search for and find further studies (such as Kampen et al) that will be included in updates.
Meanwhile, if you have comments, if you have other studies to be included, and especially if you have been diagnosed as infected or infectious please send them to tom.jefferson@conted.ox.ac.uk.
We will read all comments but we cannot promise to respond.
Source : Are you infectious if you have a positive PCR test result for COVID-19? – CEBM